 So I’ve got a little challenge for all you budding alchemists out there…
So I’ve got a little challenge for all you budding alchemists out there…
I’ve got a bit of a conundrum. When creating a Penny Portrait, it’s fairly easy to make pennies shinier (any acid will do the trick) but I’ve yet to find a way to artificially speed up the hands of time and make pennies darker…
Ideally, for a Penny Portrait you need a lot of chocolaty dark colored pennies but they can often be tricky to find. It would be fantastic if there were a way to speed up the chemical process that causes pennies to darken naturally.
So if there any students/teachers/hobbyists out there who want to take on the challenge, I’ve got a $50 prize and a free Penny Portrait Kit for you. I can imagine this would be the ideal project for a student who is stuck trying to figure out a unique and clever science fair project.
Here are the rules:
1. Only safe, household chemicals/materials may be used.
2. The process should take no longer than 2 weeks to transform a shiny penny into a darker colored penny. (Obviously, the less time it takes the better.)
3. I’m looking for the end result to be a dark brown penny. (Not black) See the image above as an example of what I’m looking for.
4. You need to be able to darken a bunch of pennies. (The poster needs about 300.)
That’s about it. First person to e-mail me a solution at info@PennyPortrait.com wins the prize! (I’ll post the winner in the comments below. If no winner has been posted, the contest is still open.)
One thought I’ve had is that since we know acids like vinegar can reverse the chemical reaction that causes a penny to turn dark, perhaps the opposite of an acid (a base) can be used to speed up the oxidation process?
Additionally, there are some products on the market that are used to darken copper: Brimstone Coin Darkener, Deller’s Darkener, and I know folks in the jewelry trade use something called “Liver of Sulfur”. One ingredient all these have in common is sulfur, so maybe that can be a starting point. I know egg whites are a great source of sulfur, so something to think about…
I’m including a link to a great article on why copper coins change color to get you started on your research:

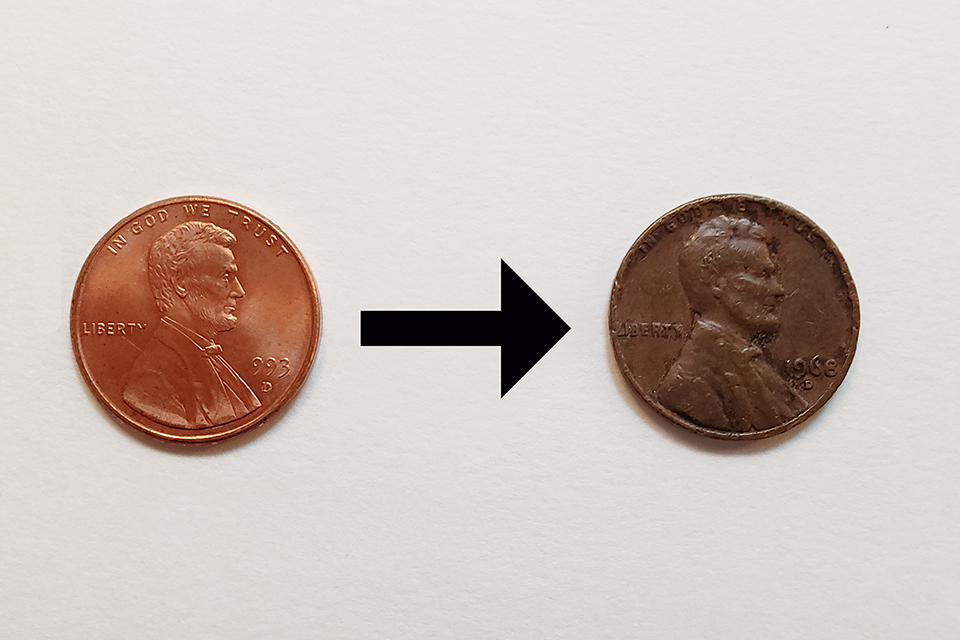

Pennies darken over time due to reaction of copper with oxygen in the air. Then they can become green due to further reaction with carbon dioxide. Dark pennies become shiny again when treated with acid because the dark copper oxide reacts with acid to make a copper salt, which can then dissolve off. So treatment with base is not likely to help. A few ideas I would like to suggest as starting points for a student would be to try heating pennies for an extended period to help then react with oxygen, or perhaps heating them in hydrogen peroxide.
Interesting… Off to find some hydrogen peroxide! (I have some sodium percarbonate here, so that might work as well…)
Liver of sulfur.
I’ve actually tried this. It tends to make them BLACK, as in jet black. They don’t look natural.
maybe that will solve the problem
http://nobel.scas.bcit.ca/wiki/index.php/Penny_reactions
Holy cow, you might be onto something here. I’ve never tried a chemical reaction that releases more copper into the solution to make pennies darker. Looks like I’ve got some experimenting to do to see if this could work for everyday folks who want to make a Penny Portrait. Exciting! I’ll let you know if I can get it to work.
I wonder if there is a way to darken the pennies without scratching the surface of them? Excited to experiment though. I’ve never thought about loading up a solution with Cu ions… Though I think it is the Oxygen that actually makes the outside dark. I should have paid more attention in Chemistry!